 +565 975 658
+565 975 658
 info@premiumcoding.com
info@premiumcoding.com
 Monday - Friday, 8.00 - 20.00
Monday - Friday, 8.00 - 20.00
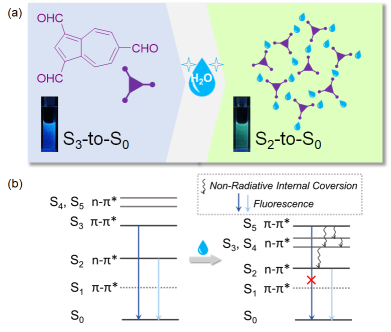
Although organic luminogens normally emit from their lowest excited state (the first excited state) according to Kasha’s rule, some anomalous cases have been found that can produce emission from higher excited states due to their ultrafast radiative rate or large S2-S1 energy gap. Recently, the exploration of this perspective is becoming prosperous (J. Phys. Chem. A., 2011, 115, 8344; Chem. Rev. 2012, 112, 4541; Chem. Sci. 2016, 7, 655; Nat. Chem. 2017, 9, 83), since these so-called anti-Kasha's rule emissions can ideally improve luminescent quantum efficiency by avoiding additional consumption from internal coversions and other forms of electronic relaxation. Thus far, scientists kept to pursue the design and development of various structures that can produce anti-Kasha's rule emissions (e. g. azulenes, thiones, pyrene, etc.) for theoretical and experimental interest (J. Am. Chem. Soc., 2011, 133, 11830; Chem. Rev., 2017, 117, 13353, etc.). However, to the best of our knowledge, the emissive transfers among these high excited states have even been poorly addressed, simply because these excited states basically bear quite small energy gaps in between. Once the emissions from different high excited states can be controlled by external stimuli, one may utilize this principle towards building luminescent sensing systems with high distinguishability.
Zhu’s group aim at seeking the strategy of employing intermolecular H-bonding to achieve a unimolecular emissive switching between different high-excited states (i.e. anti-Kasha's rule emission switching). To address the above-mentioned issues, they propose the strategy in the work with the following contributions:
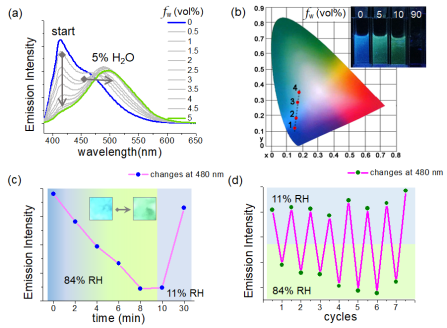
1) They presented a rational design of a novel azulene-based emitter modified by triformyl groups, in which the anti-Kasha's rule emissions can be high efficiently revealed with an outstanding fluorescent quantum yield in the azulene family. The emitter initially gave rise to S3-to-S0 dominant emission, and it can be toggled into S2-to-S0 dominant one upon the H-bond formation between the triformyl groups and water molecules. These results signify an unprecedented emissive switching of high excited states from the perspective of H-bonding regulated distribution of excited-state energy.
2) Moreover, their molecules can exhibit a remarkable fluorescent color conversion from blue to green light. Such a behavior can be exemplified into a visualized moisture sensing process both in solution and in solid film. As compared with the relevant sensing process generally achieved by certain charge transfer mechanisms based on large conjugated backbones or incorporated luminophores (Chem. Commun., 2011, 47, 4448; Phys. Chem. Chem. Phys., 2012, 14, 8803; RSC Adv., 2014, 4, 25330), their system revealed a relatively simple structure (a single azulene luminophore), generalized control fashion (a regulation by non-covalent interactions), and a well distinguishable function (strong luminescences from higher excited states) for facilitating the practical usage.
The related paper has been published in Chem Mater, with Dr. Yunyun Zhou in the group as the first author. See details:
Yunyun Zhou, Gleb Baryshnikov, Xuping Li, Mingjie Zhu, Hans Ågren, and Liangliang Zhu, Anti-Kasha's Rule Emissive Switching Induced by Intermolecular H-bonding, Chem. Mater. 2018 DOI: 10.1021/acs.chemmater.8b03699
Link: https://pubs.acs.org/doi/10.1021/acs.chemmater.8b03699
Get to know us better now!

Wechat:FDUMMers
Search!
Search across our website
Revenant @ 2018 by fudan | All Rights Reserved
Powered by Weicheng

