 +565 975 658
+565 975 658
 info@premiumcoding.com
info@premiumcoding.com
 Monday - Friday, 8.00 - 20.00
Monday - Friday, 8.00 - 20.00
As an important type of polymerization reactions, ring-opening polymerization is unique and has been widely used in synthesis of polyester using cyclic ester monomers, yet its microscopic reaction mechanism is not fully discovered. Along with their systematic research of thermogels composed of block copolymer of poly(ethylene glycol) (PEG) and biodegradable aliphatic polyesters, the group led by Professor Jiandong DING at Fudan University has recently investigated the ring-opening reaction path of the lactide monomer in stannous isooctanoate/PEG system using density functional theory.
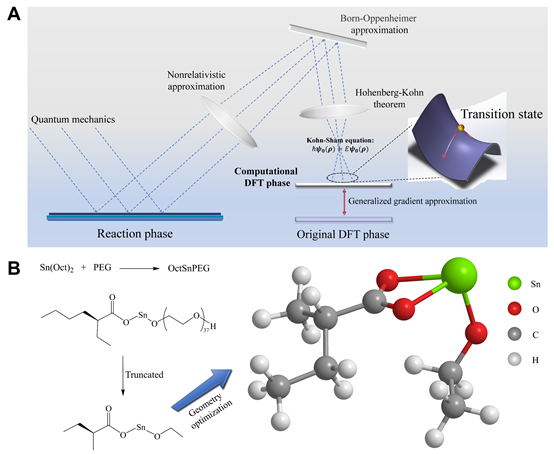
Figure 1 The reaction system modeled by density functional theory
They revealed two ring-opening pathways, which leads to the identical product. In pathway A, metal ions in catalyst firstly coordinate with the carbonyl oxygen atom of the lactide monomer, and then re-coordinate with ester oxygen atoms to trigger the ring-opening step; in pathway B, the catalyst directly triggers the ring-opening step by the coordination of the catalyst with the ester oxygen atom of lactide.
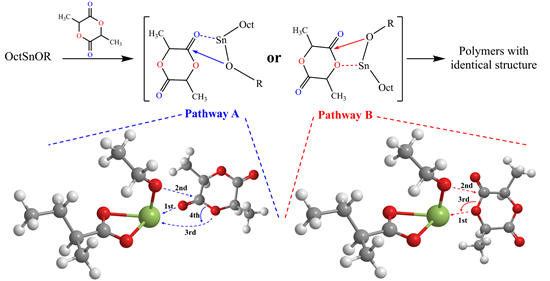
Figure 2 Two reaction pathways obtained by DFT calculation
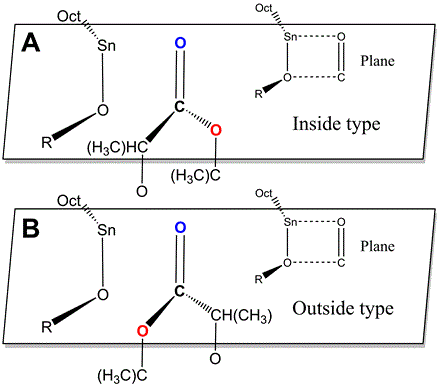
Figure 3 Turnover of lactide monomer relative to the catalyst molecule
In order to compare the two reaction paths more comprehensively, the effect of the relative spatial position of catalyst and monomer on the energy barriers of the two paths was investigated in detail. The relative spatial factors considered in this study include the turnover and the rotation of lactide monomer. Eight coordination types of pathways of the ring-opening reaction were defined, namely Outside-D type pathway A, Outside-L type pathway A, Inside-D type pathway A, Inside-L type pathway A, Outside-D type pathway B, Outside-L type pathway B, Inside-D type pathway B, Inside-L type pathway B. And the corresponding potential energy profiles were also calculated. The comparison of potential energy barriers indicated that pathway A was dominant over pathway B.
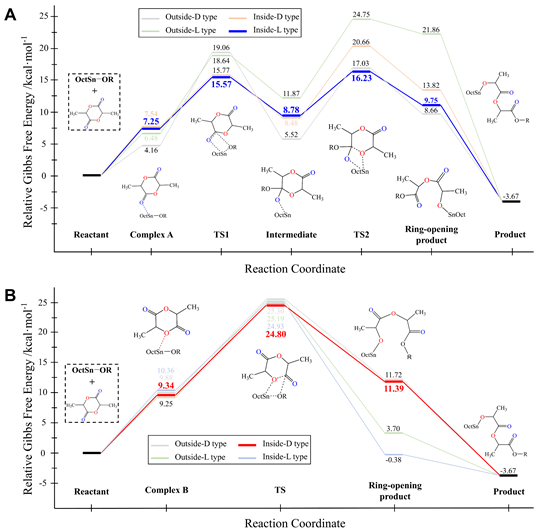
Figure 4 Potential energy profiles of eight coordination types
Furthermore, the proportion of polymerization products obtained through the two pathways was quantified with employment of Curtin Hammett principle for the first time. The research group also analyzed why pathway A is more advantageous than pathway B. In the case of pathway A, the conjugated system of ester oxygen atom and carbonyl group is destroyed by the metal ion of catalyst coordinating with the carbonyl oxygen atom of monomer, so that only one C-O single bond needs to be broken in ring-opening step, while the C-O single bond directly broken in pathway B is still in the conjugated system, the more stable molecular structure makes the ring-opening step process through pathway B much more difficult.
The result was published in Chinese Journal of chemistry. See please: Weihan Rao, Caiyun Cai, Jingyu Tang, Yiman Wei, Caiyun Gao, Lin Yu, Jiandong Ding*, Coordination insertion mechanism of ring-opening polymerization of lactide catalyzed by stannous octoate. Chinese J. Chem., 2021, 39, 1965-1974. The first author Mr. Weihan RAO is a Ph.D candidate in Department of Polymer Science, Fudan University and also The State Key Laboratory of Molecular Engineering of Polymers, and the corresponding author Professor Jiandong DING is the director of this key lab.
Article link: https://onlinelibrary.wiley.com/doi/epdf/10.1002/cjoc.202000519.
Get to know us better now!

Wechat:FDUMMers
Search!
Search across our website
Revenant @ 2018 by fudan | All Rights Reserved
Powered by Weicheng

