 +565 975 658
+565 975 658
 info@premiumcoding.com
info@premiumcoding.com
 Monday - Friday, 8.00 - 20.00
Monday - Friday, 8.00 - 20.00
Some amphiphilic block copolymers undergo a sol-gel transition upon heating. The transition temperature can be adjusted between room temperature and body temperature by chemical design, thus the material can be used as an injectable thermogel to encapsulate cells or drugs. A typical polymer is triblock copolymer PLGA-PEG-PLGA (PEG: poly(ethylene glycol); PLGA: poly(lactide-co-glycolide)).
Molecular weight distribution (MWD) is an intrinsic character of synthesized copolymers, and usually is described by molar mass dispersity (ÐM), which is defined as the ratio of the weight average molecular weight (Mw) to the number average molecular weight (Mn). Ding’s group found that ÐM from the PLGA block affected the thermogelling behaviors of PLGA-PEG-PLGA obviously. For some copolymers with very low ÐM, the copolymer might be poorly soluble and even insoluble; only copolymers with moderate ÐM could exhibit a sol-gel transition upon heating (Figure 1). The ÐM is a parameter, independent of molecular weight (MW), to regulate the thermogelation.
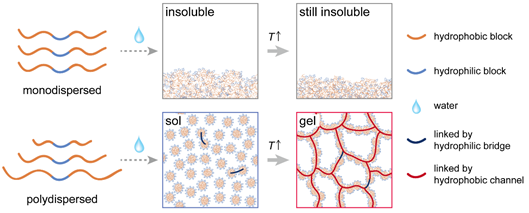
Figure 1. Schematic of ÐM effects on thermogelation
Further studies indicated that the ÐM influenced the thermogelation by affecting the corona thickness. The key to the thermogelation is the formation of the semi-bald micelle, whose prerequisite structure is the crew-cut micelle with a very thin corona. In systems with very small ÐM, the micellar corona is too thin to stabilize the micelle even at a very low temperature, thus the micelles tend to aggregate; and in an extreme case, the copolymer with a given average MW and block ratio might be insoluble in water at all. Thermogelation takes place in systems with a moderate ÐM: at low temperatures, the copolymers self-assemble to crew-cut micelles with a thin corona; then the micellar corona composed of the reversed thermosensitive PEG blocks collapses upon heating; at a high temperature, the collapsed thin corona cannot totally wrap the hydrophobic core, and the semi-bald micelle with partial exposed core areas forms; the semi-bald micelles are unstable and thus aggregate together, leading to the hydrogel network. In another extreme case of very large ÐM, the micellar corona is very thick in a star-like micelle, and even the collapsed corona can still wrap the micellar core, which hinders the formation of semi-bald micelles and the following micellar network. At very high temperatures, the excessive hydrophobicity makes the system precipitate straightforwardly.
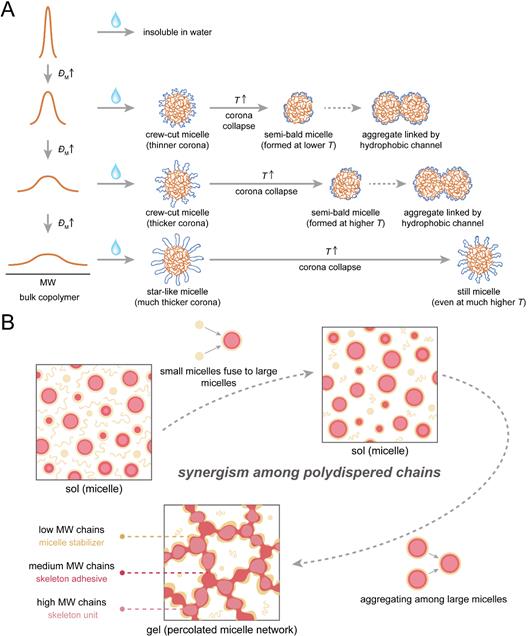
Figure 2. Mechanism of the ÐM effects on thermogelation
(A) With the increase of ÐM, the corona of the micelle gets thicker, which stabilizes the micelle and prohibits the further aggregation; (B) Schematic of the synergistic action of chains of different lengths in the thermogelling process
The research revealed the roles of components with different MW playing in the thermogelation process. The low MW chains act as the micelle stabilizer, hindering the further aggregation of the micelles. The medium MW chains act as a skeleton adhesive, which sticks the high MW chains together; the high MW chains are the skeleton unit, and the much hydrophobicity of this chain promotes the self-aggregation even at very low temperatures.
This work was published on Macromolecules with a PhD candidate student Shuquan Cui as the first author. See details: Shuquan Cui, Liang Chen, Lin Yu and Jiandong Ding*, Synergism among polydispersed amphiphilic block copolymers leading to spontaneous physical hydrogelation upon heating, Macromolecules, 53: 7726-7739 (2020)
Article links: https://pubs.acs.org/doi/pdf/10.1021/acs.macromol.0c01430
Get to know us better now!

Wechat:FDUMMers
Search!
Search across our website
Revenant @ 2018 by fudan | All Rights Reserved
Powered by Weicheng

