 +565 975 658
+565 975 658
 info@premiumcoding.com
info@premiumcoding.com
 Monday - Friday, 8.00 - 20.00
Monday - Friday, 8.00 - 20.00
All-solid-state batteries (ASSBs), comprising of solid polymer electrolytes (SPEs), represent a promising class of future lithium-ion batteries that can replace liquid-based batteries while providing safety, processability, and greater energy densities at the system level. In particular, fluorinated electrolytes have been recently shown to be interesting for lithium-ion batteries (i.e., fluorinated ether electrolytes reported by the Bao Zhenan and Cui Yi groups at Stanford, perfluoropolyether-based oligomer electrolytes reported by Joseph DeSimone group at North Carolina State University). However, traditional fluoropolymers are highly crystalline and poor ion conductors without aid of solvents. Moreover, synthesis of main-chain fluoropolymers remains a critical challenge due to demanding synthetic treatment of gaseous fluoroethylene monomers, which urges for developing high-performance electrolytes.
Recent years, PolyMao Group developed the novel photoorganocatalyzed reversible-deactivation radical alternating copolymerization of fluorinated monomers. Herein, the group reported the molecular design and synthesis of a family of alternating main-chain fluoropolymer electrolytes (MCF-SPEs) (Figure 1) that are nonflammable, amorphous, and chemically stable, which not only allow efficient lithium transport (tLi+ = 0.61), but also exhibit highly improved oxidative stability that enlarges the overall electrochemical window to 5.3 V. In addition, increasing the molecular weight of fluoropolymers facilitates Li dendrites suppression and mechanical strength. Especially for MCF-SPE(Mn = 22.8 kDa) delivers stable cycling of over 2600 operating hours in symmetric Li||Li cells with no sign of lithium dendrite proliferation.
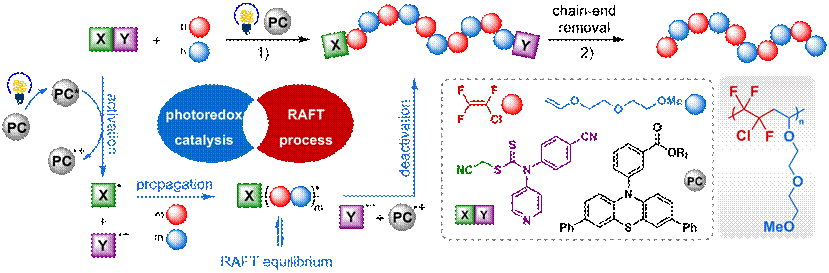
Figure 1. Synthesis of main-chain fluoropolymer and postmodification.
Furthermore, 1H−19F correlation is first analyzed via heteronuclear multiple bond correlation (HMBC) spectrum (Figure 2) to verify the F-based interactions that facilitate formation of a stable 6-membered ring structure. The interactions between Li+ and F segments facilitate the dissociation of lithium ions and binding of TFSI− anions in the solvation sheath, which devotes to the formation of anion-derived LiF-rich SEI at the anode/electrolyte interface.

Figure 2. (A) 2D HMBC spectrum of MCF-SPE. (B)Changes of chemical shift in 19F NMR for MCF-SPEs with or without LiTFSI
The corresponding work has been published on ACS Energy Letters (doi: 10.1021/acsenergylett.1c02036). The authors are Mingyu Ma, Fei Shao, Peng Wen, Kaixuan Chen, Jiarui Li, Yang Zhou, Yinli Liu, Minyi Jia, Mao Chen, and Xinrong Lin. This work is financially supported by NSFC. We also appreciate the support from Department of Macromolecular Science of Fudan University and the State Key Laboratory of Molecular Engineering of Polymers.
Article link: https://pubs.acs.org/doi/10.1021/acsenergylett.1c02036
Get to know us better now!

Wechat:FDUMMers
Search!
Search across our website
Revenant @ 2018 by fudan | All Rights Reserved
Powered by Weicheng

