 +565 975 658
+565 975 658
 info@premiumcoding.com
info@premiumcoding.com
 Monday - Friday, 8.00 - 20.00
Monday - Friday, 8.00 - 20.00
Biodegradable stents are regarded as the next generation of the corresponding interventional medical devices. Among the three candidate raw materials (polylactide, magnesium alloy and iron alloy), iron-based materials have the best mechanical strength, but their slow degradation get to be the bottleneck. In this work, a metal-polymer composite strategy was put forward, and polylactide (PLA) was coated on iron to enable the biodegradation rate of the stent match with the vascular remodeling, as shown in Figure 1. In addition, the effects of serum on scavenging reactive oxygen species (ROS) produced in iron corrosion were examined. The safety and efficacy of the metal-polymer composite stent (MPS) were verified by animal experiments and a clinical research.
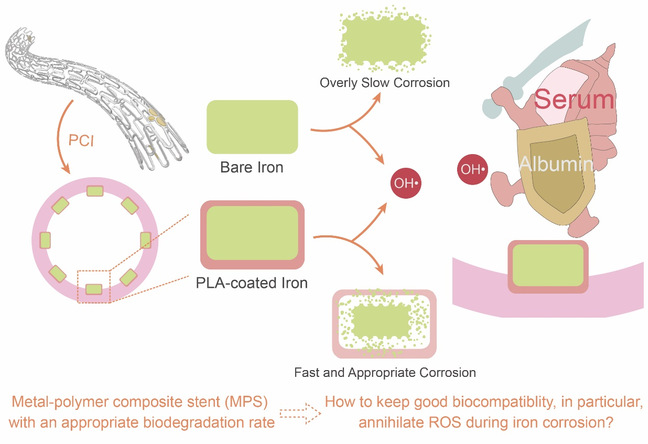
Fig. 1 Schematic diagram of the function of a PLA coating to accelerate iron corrosion in metal-polymer composite stent (MPS), and the hypothesized antioxidant capacity of the serum and albumin in the plasma for MPS as examined in this study.
In order to illustrate the unexpected characteristics of the composite strategy, the authors first prepared the PLA coating with an IRON pattern on the surface of the iron sheet via ultrasonic spraying, and immersed it in Hank’s solution, a classic medium to mimic plasma. As shown in Figure 2A, corrosion products accumulated only in the area covered by the PLA coating during the observation time, which demonstrated that the PLA coating could accelerate iron corrosion instead of slowing it down. The corrosion products were quantified by ICP-AES, and it can be seen from Figure 2B that the PLA coating significantly increased the corrosion amount of iron, strengthening that the metal-polymer composite strategy did increase the degradation rate of iron.
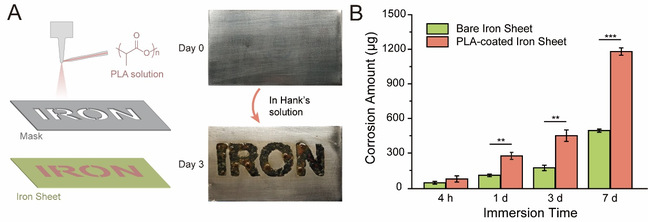
Fig. 2 PLA coating accelerates iron corrosion in Hank’s solution. (A) Illustration of the iron sheets with the PLA coating of an “IRON” pattern experiencing ultrasonic spraying of a PLA solution thorough a mask (left), and the optical photographs of the iron sheets before and after being immersed in Hank’s solution for 3 days (right). (B) The corrosion amounts of the PLA-coated iron sheet and bare iron sheet after being immersed in Hank’s solution at 37 °C for 4 hours, 1 day, 3 days, and 7 days.
In order to explore the effect of serum on oxidative damage along with iron corrosion, human umbilical vein endothelial cells (HUVECs) were cultured in DMEM containing 0.8 g/L carbonyl iron powder and different concentrations of fetal bovine serum (FBS). The groups without carbonyl iron powder were set as controls, and the cell viability was characterized by CCK-8 kit. As seen from Figure 3A, carbonyl iron powder had no effect on cell viability when only 0.5% FBS was added, indicating that serum could reduce the oxidative damage of cells caused by iron corrosion significantly. The results in Figure 3B illustrate that albumin in serum plays an important role in reducing oxidative damage. In addition, the authors found that the serum and albumin worked mainly via scavenging the hydroxyl radicals.
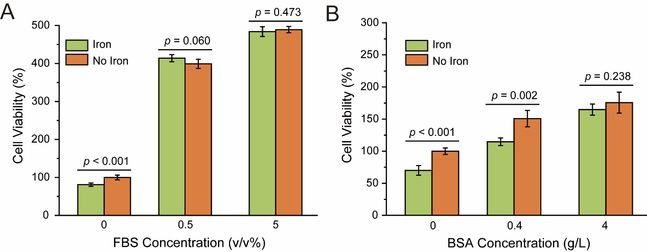
Fig. 3 The oxidative damage to cells caused by iron corrosion was reduced by the serum and albumin in the plasma. (A) Fetal bovine serum (FBS) and (B) bovine serum albumin (BSA), the analogue of serum and albumin respectively, reduce the oxidative damage to HUVECs.
The in vitro experiments revealed that the metal-polymer composite strategy accelerated iron degradation and the serum ensured the biosafety of iron-based materials. Then the MPS was fabricated and implanted into the coronary arteries of mini-pigs, with the currently gold standard of the coronary stent Xience as a control (the matrix of Xience is nondegradable cobalt-chromium alloy). As shown in Figure 4, all the stented coronary artery remained patent and no thrombosis were observed in both groups by coronary angiography. From the results of micro-CT, MPS degraded slightly at 6 months and almost half degraded at 2 years. The appropriate biodegradation rate not only avoided the slight loss of mechanical integrity at the early stage, but also circumvent chronic inflammatory owing to the temporary caging. The results were further confirmed by optical coherence tomography (OCT).

Fig. 4 (A) Schematic diagram of the design and implantation of the metal-polymer composite stent (MPS). (B) The coronary angiography images of the porcine coronary artery at 1, 3, 6 months and 1, 2 years after the implantation of MPS or Xience. (C) The micro-computed tomographic (micro-CT) images of the stented coronary artery at each follow-up point.
The biosafety of MPS was further characterized by hematoxylin and eosin (H&E) staining. ROS can induce oxidative damage to surrounding tissues, then contribute to inflammation and restenosis. Yet there was no statistically significant difference in either inflammation scores or area stenosis between the biodegradable stent MPS and the permanent stent Xience, indicating that the biodegradation of MPS had no damage to surrounding tissues owing to the good antioxidant capacity of serum. The results were further confirmed by OCT, which further demonstrated the safety of MPS stents in vivo.
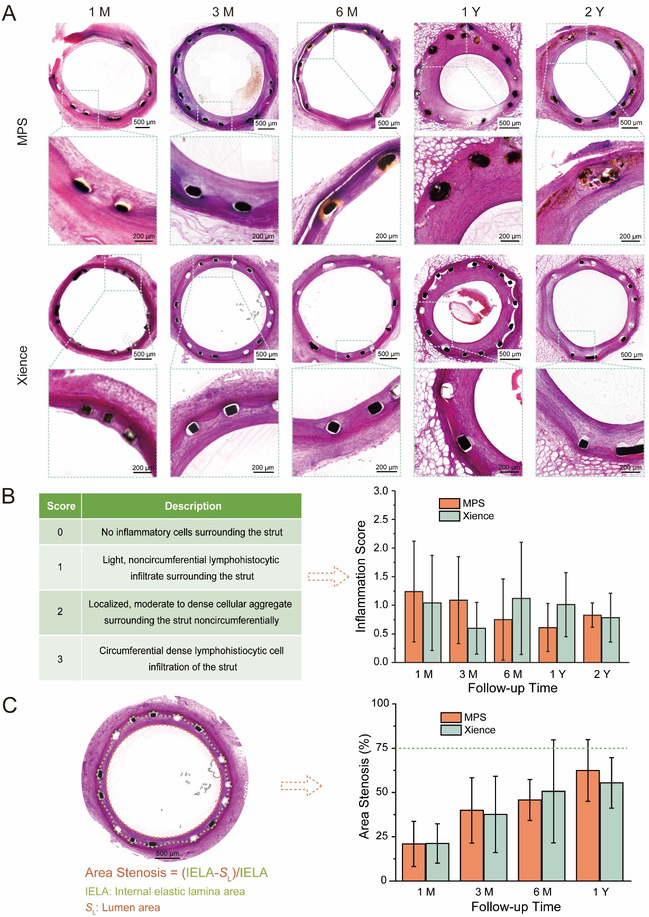
Fig. 5 (A) Hematoxylin and eosin (H&E) staining images of the porcine coronary artery 1, 3, 6 months and 1, 2 years after the implantation of MPS or Xience. (B) The criteria for evaluation of inflammation scores by H&E staining images (left), and the inflammation scores of the stented coronary artery at each follow-up point (right). (C) Schematic diagram of the area stenosis calculated by H&E staining images (left), and the area stenosis of the stented coronary artery at each follow-up point (right).
Finally, the first clinical implantation of MPS was carried out via percutaneous coronary intervention with the relevant results shown in Figure 6. The struts began to degrade at 6 months after the implantation and showed a high degree of degradation at 26-month follow-up. The stented coronary artery remained patent and no thrombosis was observed, which proved the biosafety, efficacy and excellent degradation performance of MPS.
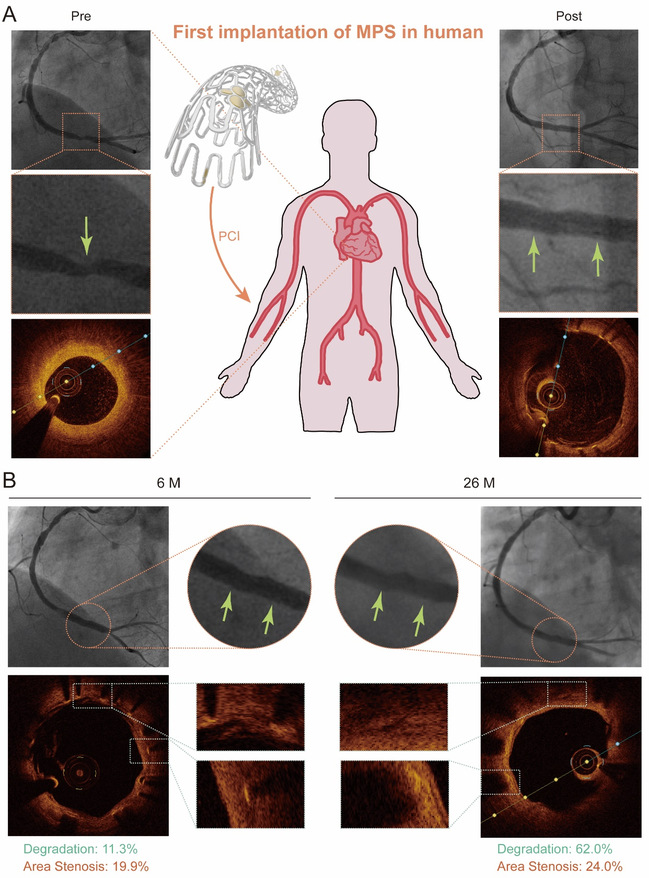
Fig. 6 (A) Schematic diagram of the first implantation of MPS into a 60-year-old man, and the coronary angiography images and the OCT images of the right coronary artery before (left) and immediately after percutaneous implantation (right). (B) The coronary angiography images and the OCT images of the right coronary artery stented by MPS 6 months (left) and 26 months (right) after implantation.
In conclusion, a metal-polymer composite strategy is applied to control the degradation of iron-based materials. The stents fabricated with this strategy exhibit a balance between accelerated biodegradation and adequate radial strength to match the arterial remodeling. This work highlights the ability of serum, especially the albumin in it, to scavenge ROS and thus enhance the biosafety of iron-based materials. The first implantation of MPS in human and animal experiment further demonstrated its safety and efficacy, supporting its following clinical trials.
It should be pointed out that a paper entitled as “Hydroxyl radicals and oxidative stress: the dark side of Fe corrosion” was published by Scarcello et al (ref. 38). They declared that the so-called standard ISO to assess the cytotoxicity of a biomedical device using extract medium is inappropriate for iron-based materials. After putting iron powder to directly contact with cells, they found that the oxidative stress caused by iron corrosion was detrimental to endothelial cells significantly. This is a serious problem related to any effort of an iron-based biomaterial eventually implanted into body. Ding’s group confirmed the reproducibility of the experiments, and further found that the authors in ref. 38 ignored the effect of serum to scavenge the free radical. According to the well-designed experiments by Ding et al., the oxidative damage was negligible with only 0.5 v/v% serum, which was further confirmed by animal experiments.
Professor Jiandong Ding points out in their paper that “In the science history, a ‘dark side’ can sometimes trigger a new progress”. Scientific debate based on facts is an important source of scientific and technological progress. From the perspective of basic research, this paper, which stems from the development of novel biodegradable coronary stents, highlights the importance to select an appropriate biomimetic medium to assess a medical implant in a physiological condition, especially if one examines ROS generation and pertinent possible “dark side” of any biomaterial in contact with blood.
The study was published in Advanced Healthcare Materials, an international journal of biomedical materials. The first author is Mr. Hongjie Zhang, a Ph. D. student in the State Key Laboratory of Molecular Engineering of Polymers, Department of Macromolecular Science, Fudan University. Professor Jiandong Ding (his supervisor) at Fudan University, Academician Runlin Gao at Fuwai Hospital and Dr. Deyuan Zhang at Biotyx Medical (Shenzhen) Co., Ltd. are co-corresponding authors.
Hongjie Zhang, Wanqian Zhang, Hong Qiu, Gui Zhang, Xin Li, Haiping Qi, Jingzhen Guo, Jie Qian, Xiaoli Shi, Xian Gao, Daokun Shi, Deyuan Zhang*, Runlin Gao*, Jiandong Ding*. A biodegradable metal-polymer composite stent safe and effective on physiological and serum-containing biomimetic conditions.Advanced Healthcare Materials, 2201740 (2022)
https://onlinelibrary.wiley.com/doi/abs/10.1002/adhm.202201740
Get to know us better now!

Wechat:FDUMMers
Search!
Search across our website
Revenant @ 2018 by fudan | All Rights Reserved
Powered by Weicheng

