 +565 975 658
+565 975 658
 info@premiumcoding.com
info@premiumcoding.com
 Monday - Friday, 8.00 - 20.00
Monday - Friday, 8.00 - 20.00
Photoinduced electron transfer plays an extremely important role in physics, materials science, molecular biology and synthetic chemistry, and almost all organic photocatalysts are based on the outer-sphere electron transfer to achieve redox reactions. Xiangcheng Pan's research group proposed to use the special chemical properties and high electron deficient characteristics of boron atoms to construct an organoboron ion pair. This compound is able to form a complex with the counter ion pair in the excited state, realizing the inner-sphere electron transfer within the ion pair under the photoexciation, which is conducive to realizing the electron transfer reaction of thermodynamic unfavorable. Therefore, this property can be used as a means to generate various types of free radicals (such as chlorine radicals, sulfur-containing radicals, carbon radicals and oxygen radicals, which play an indelible role in the catalytic field).
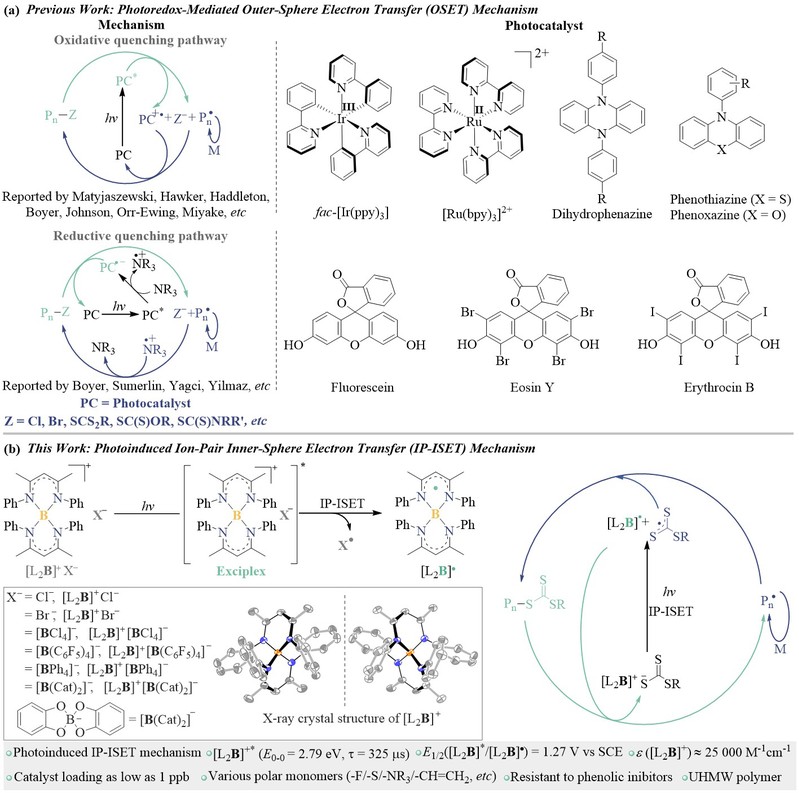
Figure 1. Inner-sphere electron transfer for controlled radical polymerization.
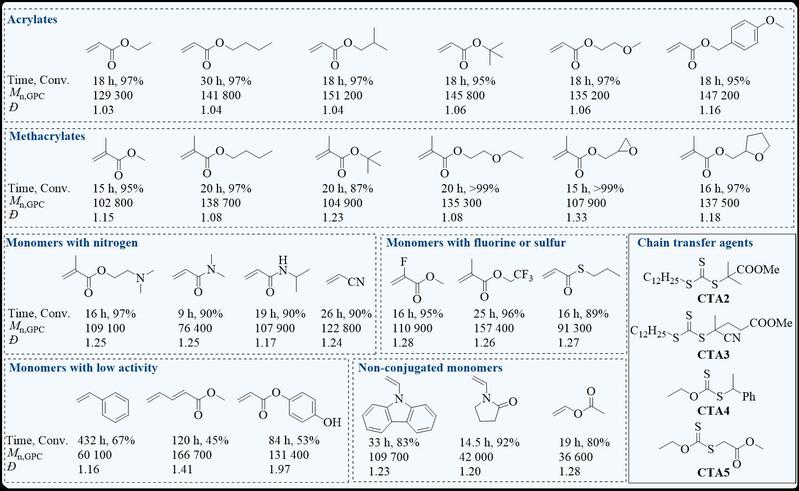
Figure 2. The monomer scope and the polymerization results of organoboron catalyzed inner-sphere electron transfer for controlled radical polymerization.
This photocatalyst can be applied to RAFT polymerization with the amount of catalyst as low as 1 ppb, and the obtained polymer has the molecular weights consistent with the theoretical and maintains a narrow distribution. The mechanism of this organoboron photocatalyst is that ion-pair electron transfer occurs firstly under photoexcitation, which is different from the traditional photocatalytic controlled radical polymerization. The ion-pair electron transfer mechanism and the formation of radical [L2B]• are supported by electron paramagnetic resonance (EPR) radical capture experiments, 1H/11B NMR, spectroelectrochemical spectra, transient absorption spectra, theoretical calculation and photoluminescence quenching experiments. Photoluminescence quenching experiments show that when [CTA]/[[L2B]+] ≥ 0.6, it is static quenching because of the in-situ formation of [L2B]+[ZCS2]–, the real catalytic species. The [L2B]+[C3H7SCS2]– is synthesized, and its photoluminescence lifetime is the same as the lifetime in static quenching experiment, indicating the formation of [L2B]+[ZCS2]– in polymerization and the IP-ISET mechanism. Matrix-assisted laser desorption ionization time-of-flight mass (MALDI-TOF MS) spectra show that the structure of [C3H7SCS2] is incorporated into the polymer, indicating that ion-pair electron transfer occurs in catalytic species. Owning to this specific ion-pair electron transfer mechanism, the polymerization can accommodate a variety of monomers with functional groups, such as the amine group that is easy to be oxidized, the phenolic hydroxyl group that has an inhibitory effect on polymerization, and non-conjugated monomers.
This work was recently reported by Xiangcheng Pan's research group from the Department of Macromolecular Science and the State Key Laboratory of Molecular Engineering of Polymers at Fudan University with a title on 'Photoinduced Ion-Pair Inner-Sphere Electron Transfer-Reversible Addition–Fragmentation Chain Transfer', published online on October 20, 2022 in J. Am. Chem. Soc.. The first author of this paper is Dr. Qianyi Wang, a postdoctoral fellow at Fudan University, and Prof. Pan is the corresponding author of the paper. The State Key Laboratory of Molecular Engineering of Polymers, Department of Macromolecular Science, and Fudan University, is the first unit of the paper. Fengyang Bai and Yiyan Zhang of Shenyang Normal University, Ke Hu of Fudan University and Qixi Mi of ShanghaiTech University also participated in the study. This research was supported by the National Natural Science Foundation of China, the Natural Science Foundation of Shanghai, and Fudan University.
Paper Link: https://pubs.acs.org/doi/10.1021/jacs.2c08173
Introduction of the XPan research group
Prof. Xiangcheng Pan's research group has long been committed to the research of controlled and precise synthesis of polymers and the sustainable development of polymers. In recent years, a series of results have been achieved for the 'controlled and precise synthesis of polymers based on heteroatoms': orgonoboron (J. Am. Chem. Soc.2022, 10.1021/jacs.2c08173; Nat. Commun.2021, 12: 5853; Angew. Chem. Int. Ed.2018, 57, 9430; Macromolecules2020, 53, 3700; 2021, 54, 6000; ACS Macro. Lett.2021, 10, 1327; Cell. Rep. Phys. Sci.2020, 1, 100073) and silicones (J. Am. Chem. Soc.2021, 143, 19167). Students interested in the research direction of the research group are welcome to contact and consult the opportunity to join the research group (including summer camp students, master's and doctoral students, and postdoctoral fellows).
Research group website: www.panxlab.com
Get to know us better now!

Wechat:FDUMMers
Search!
Search across our website
Revenant @ 2018 by fudan | All Rights Reserved
Powered by Weicheng

