搜索

细胞与材料相互作用是一个重大的基础科学问题。以往的国际主流学术观点认为,稳定的细胞黏附导致更高的细胞骨架张力是促进细胞成骨分化的关键。然而,复旦大学丁建东课题组之前基于其创建的高分子水凝胶表面特异性细胞黏附短肽序列精氨酸-甘氨酸-天冬氨酸(RGD)纳米图案的精准控制技术,发现大的RGD间距虽然不利于细胞黏附、却促进了骨髓基质干细胞(MSC)的成骨分化。对于这一“反常”现象的解释催生了进一步的基础研究,通过探讨生物材料表面的纳米信号如何传导到细胞内部、并调控干细胞分化,最终解释了一个生物力学相关的信号传导机制,表明细胞核内肌动蛋白的核转位及其聚合形成的核张力是激活成骨基因的重要驱动因素。
研究团队利用嵌段共聚物胶束纳米光刻技术,精准制备了模量为1.3 MPa、RGD黏附配体间距分别为30纳米与150纳米的纳米图案化水凝胶材料。实验结果显示,在150纳米大间距的条件下,细胞铺展面积缩小、黏着斑明显缩小,同时II型肌球蛋白磷酸化水平降低、肌动蛋白骨架张力下降。然而,出人意料的是,在这种骨架张力下降的状态下,早期成骨标志物osterix、碱性磷酸酶(ALP)活性以及成骨基因RUNX2、IBSP的表达却显著提升。
为了破解这一“低细胞力—高成骨活性”的谜团,研究团队从多个角度进行了探索,最终将目光投向了细胞核。他们发现,在大间距条件下,细胞黏附失稳,加速了肌动蛋白骨架(一种超分子聚合物)在细胞质内的“组装—解离”动态过程,产生了大量的球状肌动蛋白单体。这些肌动蛋白单体通过内输蛋白importin 9被转运入核,并在细胞核内重新聚合形成纤维状肌动蛋白,从而显著提升了细胞核的张力。增强的核张力进一步促进了核骨架蛋白lamin A/C的组装,改变了核形态(如核高度和体积增加)及染色质结构,使得染色质松散、组蛋白乙酰化水平升高;并诱发了内质网钙离子释放,这些都为成骨基因的转录提供了有利条件。
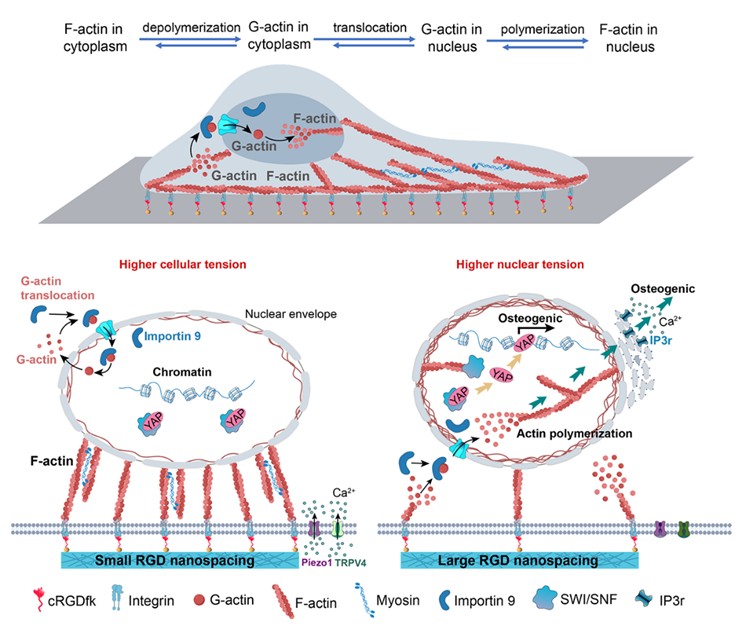
生物材料表面纳米信号通过细胞膜、细胞质向细胞核的传导机制的示意图,其中的一个关键在于肌动蛋白这个生物大分子在细胞核内的自组装
可见,细胞核内的力学状态——核张力的变化——在调控干细胞命运中起着至关重要的作用。通过调控纳米尺度的细胞黏附配体的纳米级空间分布,可实现对细胞核力学状态的精准调控,从而更有效地诱导成骨分化。这一发现丰富了再生生物材料的基础理论,也对生物材料的界面设计提供了新的视角。
数届研究生和博士后参与了这个交叉研究。这个基于长期探索而凝练的论文在国际权威综合性学术期刊《美国国家科学院院刊》(PNAS)上发表,由刘晓静博士后、张曼博士后、王鹏博士后为共同第一作者,复旦大学和四川大学等单位多个年级的硕士生和博士生等为共同作者,复旦大学聚合物分子工程全国重点实验室主任丁建东教授和该全国重的访问学者、四川大学魏强研究员为共同通讯作者。
论文信息:
Xiaojing Liu#, Man Zhang#, Peng Wang#, Kaikai Zheng, Xinlei Wang, Wenyan Xie, Xiaokai Pan, Runjia Shen, Ruili Liu, Jiandong Ding*, Qiang Wei*. Nanoscale distribution of bioactive ligands on biomaterials regulates cell mechanosensing through translocation of actin into nucleus. Proc. Natl. Acad. Sci. USA. 122(10), e2501264122 (2025).
论文链接:https://www.pnas.org/doi/10.1073/pnas.2501264122
Uncover the Nuclear Code for a Biomaterial Surface to Regulate Stem Cell Differentiation Using Nanopatterning Techniques
Cell-material interactions are critical for advanced biomaterials and regenerative medicine. According to the classic viewpoint, stable cell adhesion leads to higher cytoskeletal tension and thus promotes osteogenic differentiation of mesenchymal stem cells (MSCs). Its universality was, however, challenged by Prof. Jiandong Ding at Fudan University, for his team observed an anomalous phenomenon based on well-controlled nanopatterns of cell-adhesive arginine-glycine-aspartic acid (RGD) on nonfouling polymeric hydrogels. They found that large RGD spacings hindered cell adhesion yet sometimes promoted osteogenic differentiation of MSCs. This unexpected phenomenon prompted extensive research to explore how nanoscopic surface signals are transduced into the cells and regulate stem cell differentiation, eventually leading to the identification of a biomechanics-related signaling pathway. They demonstrated that nuclear translocation of actins and their polymerization into filamentous structures in cellular nucleus, increasing nuclear tension, play a pivotal role in activating osteogenic genes.
The research team employed block copolymer micelle nano-lithography to precisely fabricate hydrogels with modulus values of 1.3 MPa and RGD ligand spacings of 30 nm and 150 nm. MSCs on the nanopatterns of 150 nm nanospacing exhibited reduced spread areas, smaller focal adhesions, and decreased phosphorylation levels of myosin II, along with diminished actin cytoskeletal tension. Surprisingly, despite these changes, early osteogenic markers such as Osterix, alkaline phosphatase (ALP) activity, and the expression of osteogenic genes RUNX2 and IBSP were significantly upregulated.
To uncover the mechanism behind this low cytoskeletal tension—high osteogenic activity paradox, the team conducted a series of experiments and ultimately ascertained that the “code” was hidden in the cell nucleus. Under large spacing conditions, unstable cell adhesion accelerated the dynamic assembly-disassembly process of actin filaments, generating a substantial amount of globular actin monomers. These monomers were transported into the nucleus via importin 9, where they polymerized into filamentous actin, significantly enhancing nuclear tension. Increased nuclear tension further promoted the assembly of nuclear lamins A/C, altering nuclear morphology (e.g., increased nuclear height and volume) and chromatin structure, leading to chromatin decondensation and elevated histone acetylation levels. This process also triggered calcium release from the endoplasmic reticulum, facilitating transcription of osteogenic genes.
This fundamental study demonstrates that the mechanical state within the cell nucleus—nuclear tension—plays a crucial role in regulating stem cell fate. By manipulating nanoscale adhesive ligand distributions, the mechanical properties of the cell nucleus can be precisely tuned to more effectively induce osteogenic differentiation. This discovery not only enriches the theoretical foundation of regenerative biomaterials but also offers a new perspective for the design of biomaterial interfaces.
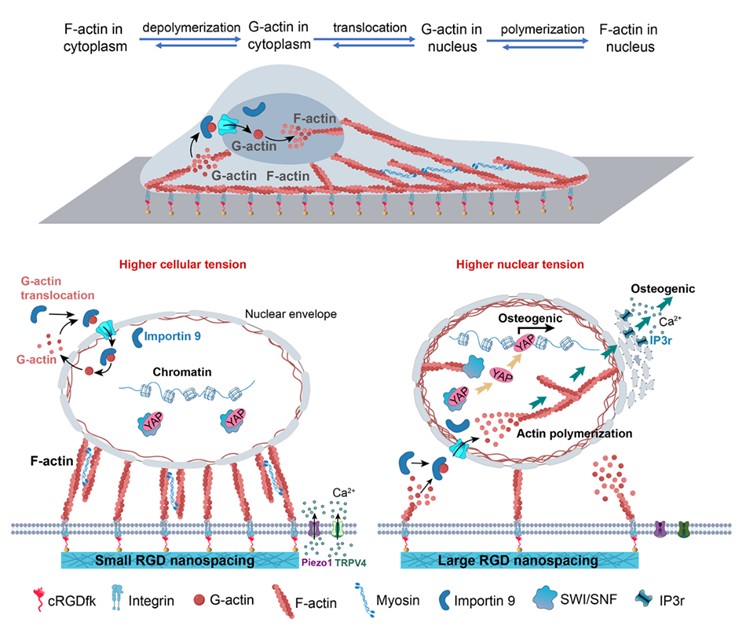
A schematic diagram of the mechanism by which nanoscopic signals on biomaterial surfaces are transduced through the cell membrane and cytoplasm to the cell nucleus, where a key event is self-assembly of nuclear actin.
The research article has been published in the journal Proceedings of the National Academy of Sciences of the United States of America (PNAS). The first authors of the paper are three postdoctors (Dr. Xiaojing Liu, Dr. Man Zhang, and Dr. Peng Wang), with contributions also from graduate students at Fudan University, Sichuan University and other institutes for years. Dr. Jiandong Ding, a distinguished professor at Fudan University and the director of the State Key Laboratory of Molecular Engineering of Polymers, and Dr. Qiang Wei, a professor at Sichuan University and also a visiting researcher of the key laboratory, are the corresponding authors.
Reference:
Xiaojing Liu#, Man Zhang#, Peng Wang#, Kaikai Zheng, Xinlei Wang, Wenyan Xie, Xiaokai Pan, Runjia Shen, Ruili Liu, Jiandong Ding*, Qiang Wei*. Nanoscale distribution of bioactive ligands on biomaterials regulates cell mechanosensing through translocation of actin into nucleus. Proc. Natl. Acad. Sci. USA. 122(10), e2501264122 (2025)..
Link:https://www.pnas.org/doi/10.1073/pnas.2501264122




